| Subatomic Particle | Relative Charge | Relative Mass / a.m.u |
|---|---|---|
| Protons | +1 | 1 |
| Neutrons | 0 | 1 |
| Electrons | -1 | 1/1840 |
Protons, Neutrons and Electrons
- Most of the mass is concentrated in the nucleus
- An atom is electricaly neutral since they have the same number of protons and electrons; P = e-
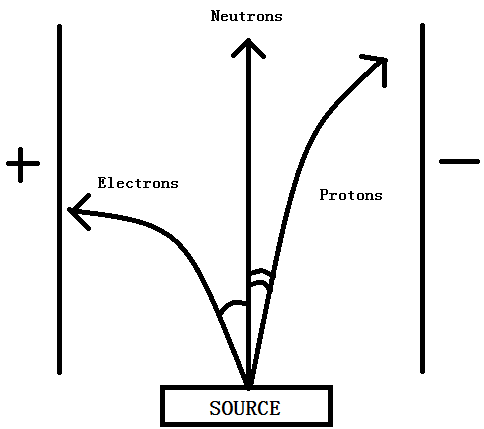
Behaviour of a beam of Subatomic Particles
- Protons, positively charged, deflected to negative pole
- Neutrons, no charge, neutral
- Electrons, negatively charged, deflected to positive pole ( deflected at a greater angle since electron is lighter than proton )
Tip
Quite similar to types of radiation in IGCSE Physics, alpha, beta and gamma. They are each attracted to a pole
